




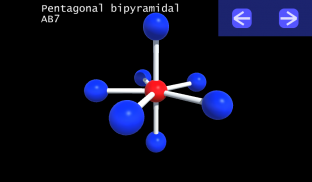
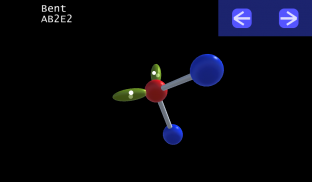
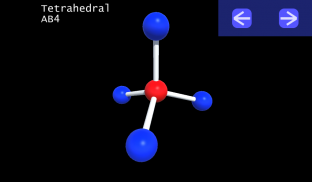
3D VSEPR

Опис програми 3D VSEPR
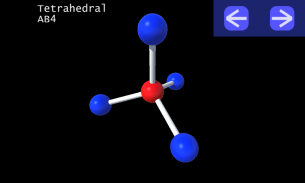
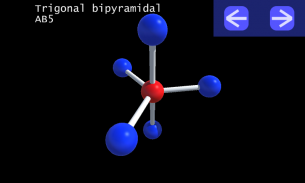
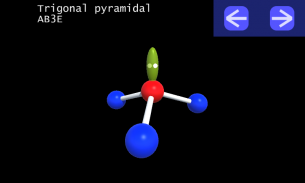
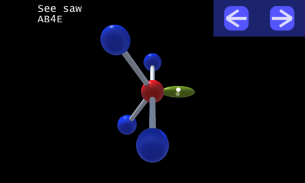
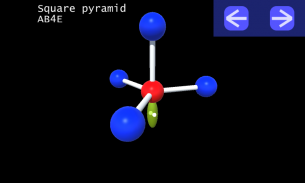
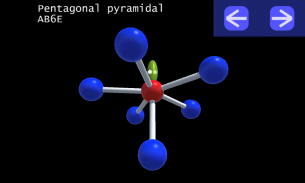
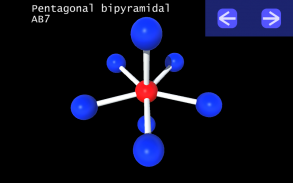
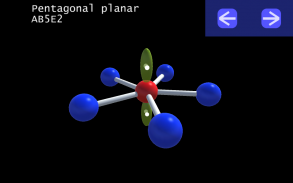
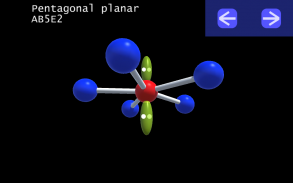
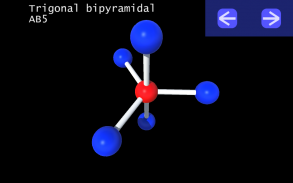
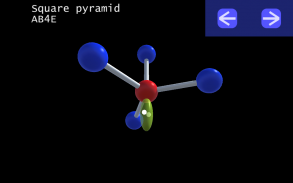
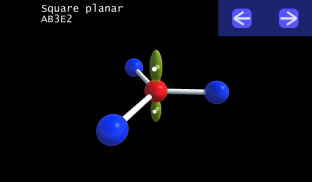
It is impossible to learn about the shapes of VSEPR models in a page which is 2D because these aren't in 2D. 3D VSEPR app helps you to visualize the shapes of the VSEPR models in 3D such that you can understand more and you can sort out your confusions.
This education app help the students to learn chemistry in a smarter way. Students can see the every parts of models by swiping their fingers to screen.
Tag: Learning App, Make Chemistry Easy, Smart Education, Class 11, Class 12, Class XI, Class XII, 3D Chemistry, 3D Orbitals, Orbit, Virtual Study, VSEPR Theory, VSEPR Animation, VESPER, VESPR, App for Smart Classes, Orbital, Shape of orbitals, Atom, orbitals of all atoms, Basic of Chemistry, SPDF, S Orbital, P Orbital, D Orbital, F Orbital, How orbitals looks, atomic orbitals, Shapes of atomic orbitals, theory of orbitals, structure of atom, sub-shell, sub shells, Filling of orbitals in atom, electronic density, arrangement of electrons in atoms, arrangements of orbitals, where are electrons, electron, electronic configuration of atoms, see orbitals, observe orbitals, what are the shapes of orbitals, understanding orbitals, chemistry lovers, micro world, microscopic world, microscopic education, futuristic education, where electrons exists, molecular orbital theory, quantum physics, quantum numbers, molecular orbital diagrams, orbital diagrams, chemistry app, VSEPR Theory, Molecular Orbital Theory, h bond, h-bond, basic chemistry, organic chemistry, chemical bonding, Periodic Table, PCM, PCB, PCBM, 3D Molecules, quantum mechanism, shapes of molecules, chemistry education app.
Atoms included: Hydrogen, Helium, Lithium, Boron, Carbon, Oxygen, Neon, Sodium, Silicon, Potassium, Argon, Calcium, Zinc, Iron etc.

























